Infectious Waste Sharps Disposal Options
Campus laboratory safety inspectors have noted increasing inconsistencies regarding how certain sharps waste are being managed in campus laboratories. At issue is how hard plastic serological, glass Pasteur pipettes, pipette tips and hard plastic Petri plates are being packaged and handled through disposal.
Environmental Health and Safety (EHS) has worked over the years with the Indiana State Board of Health and the Marion County Public Health Department to develop alternative disposal procedures in lieu of hard-plastic, single-use, disposable sharps containers in an effort to provide an appropriate level of worker safety while providing for significant cost savings. The following alternative disposal options have been approved by both regulatory agencies (Please note that these procedures are not approved for higher-hazard sharps waste such as metal lancets, scalpel blades, syringes or needles).
Hard Plastic or Glass Serological Pipettes -
Reusable Pipette Trays Containing Chemical Disinfectant
One alternative to using a hard plastic, single use, disposable sharps container is to follow the guidelines below in their entirety. Once generated, the contaminated hard plastic or glass serological pipette is to be placed into a hard plastic or metal, reusable pipette tray filled to an appropriate level with a fresh solution of an EPA-approved disinfectant. This decontamination solution must be appropriate for the biological agents used in the lab. Please check the approved IBC protocol for your lab or ask our Biosafety staff to identify an appropriate disinfectant. The tray is required to have a tight-fitting lid which must be labeled with a universal biohazard label or embossed with a universal biohazard symbol. Vinyl stick-on labels will suffice for this purpose provided the labels are changed as they become damaged or are no longer clearly visible. The pipette tray is to be covered when not actively being used.
Following treatment (for the required contact time specified for the disinfectant used), the disinfectant solution is to be decanted off and the treated pipettes transferred to any standard corrugated cardboard box lined with a plastic bag. (The box does not need to be a commercially-available infectious waste or glass waste box. Any standard corrugated cardboard box will suffice).
The box is to be taped closed and labeled with the following information:
CONTAINS TREATED BIOHAZARD WASTE MAY CONTAIN BROKEN GLASS Building and Room Number of Generation Contact Telephone Number
Building Services or laboratory staff are then responsible for collecting the waste from each lab and providing final disposal by placing in the building dumpster/trash compacter unit.
Hard Plastic Serological Pipettes, Petri Plates or Other Similar Wastes -
Reusable Tub (or other suitable container) Followed by Autoclaving
Please note the following guidelines are not appropriate for biohazardous glass waste. At the point in time when an applicable sharp item is generated, the item is to be placed into a hard plastic, reusable hard-plastic, thick-gauged plastic tub or other suitable container. Five-gallon Nalgene plastic tubs have commonly been used for this purpose. At the discretion of the lab, the tub can be lined with a biohazard bag or other plastic bag capable of withstanding autoclave temperatures to help facilitate removal of the contents following treatment. The container is required to have a tight-fitting lid which must be labeled with a universal biohazard label or embossed with a universal biohazard symbol. Again, vinyl stick-on labels will suffice for this purpose.
Following autoclaving, the treated waste is to be transferred to any standard cardboard box. In the event an autoclave bag is used to line the tub, the bag can be tied-off and transferred to the corrugated cardboard box following treatment.
The box is to be taped closed and labeled with the following information:
CONTAINS TREATED BIOHAZARD WASTE Building and Room Number of Generation Contact Telephone Number
Glass Pasteur Pipettes
Another alternative to using a hard plastic, single use, disposable sharps container is to follow the guidelines below in their entirety. Glass Pasteur pipettes can be placed into paperboard pipette keepers labeled with a universal biohazard waste as indicated below:
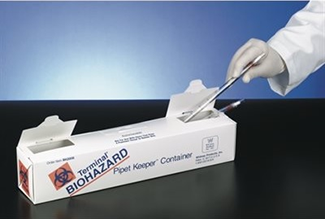
Following autoclaving, the keepers must be transferred to any standard corrugated cardboard box, the box taped closed and labeled with the following information as described previously:
CONTAINS TREATED BIOHAZARD WASTE MAY CONTAIN BROKEN GLASS Building and Room Number of Generation Contact Telephone Number
Burn-Up Boxes

EHS has evaluated a number of cardboard sharps containers commonly referred to as “burn-up boxes or bins” and found that these box systems typically provide adequate protection for hard plastic pipettes, Petri plates, culture bottles and flasks and other similar laboratory wastes. These systems are not suitable or approved for infectious wastes constructed of metal including hypodermic needles, lancets and scalpels.
The containers frequently are comprised of two cardboard boxes and an autoclaveable liner which, when assembled, create a box-within-a-box system. When properly assembled, the system readily withstands the autoclaving while remaining fully intact.
Biological testing has demonstrated that potentially-infectious wastes placed in the boxes are effectively treated by the autoclave process and that the box system does not insulate the contents from the heat of the autoclave. As there are a number of burn-up style boxes on the market, the system must be able to withstand being autoclaved while remaining fully intact in order to be considered as an acceptable alternative. 
Closing Thoughts
Laboratory personnel periodically inquire of EHS whether the waste management process can be simplified further by allowing staff to manage the types of waste described within this article exclusively in biohazard waste bags provided that only laboratory staff handle the waste from the time it is produced until the waste is physically removed from the building provided that the lab staff are willing to accept any and all potential risks involved. This is not something that EHS will authorize under any circumstance.
The University has a legal and ethical obligation to ensure that employees adhere to all applicable campus safety programs and policies. These compliance programs/policies have been developed to provide for a reasonable level of safety while meeting federal, state and/or local regulatory requirements which, with rare exception, do not allow for employee discretion.
The Office of Environmental Health and Safety has identified hard plastic pipettes and Petri plates as the infectious waste most frequently packaged incorrectly. Other labware constructed of softer plastic or items of a geometric shape that provides enhanced structural durability such as capped plastic culture bottles, flasks and centrifuge tubes have not been identified to date as creating significant safety hazards for campus personnel.
Please contact our office at ehs@iupui.edu or 274-2005 if you have any questions. |