Liquid Nitrogen Safety
 Liquid nitrogen is a commonly used cryogenic liquid in research laboratories that researchers often become familiar handling on a regular basis and can lose sight of the hazards. A “cryogenic” liquid is defined as a liquid with a normal boiling point below –130°F (–90°C). The most commonly used gases in a laboratory that are transported, handled, and stored in the liquid state at cryogenic temperatures are helium and nitrogen. Additional information about hazards of liquid nitrogen is described below: Liquid nitrogen is a commonly used cryogenic liquid in research laboratories that researchers often become familiar handling on a regular basis and can lose sight of the hazards. A “cryogenic” liquid is defined as a liquid with a normal boiling point below –130°F (–90°C). The most commonly used gases in a laboratory that are transported, handled, and stored in the liquid state at cryogenic temperatures are helium and nitrogen. Additional information about hazards of liquid nitrogen is described below:
Extremely Cold Temperatures
The temperature of liquid nitrogen is -321 degrees Fahrenheit and even the vapor of liquid nitrogen can rapidly freeze skin tissue and eye fluid, resulting in cold burns, frostbite, and permanent eye damage.
Asphyxiation
Liquid nitrogen has a high expansion rate which is 695 times in volume when it vaporizes. Nitrogen has no warning properties such as odor or color and if sufficient liquid nitrogen is vaporized it has the potential to reduce the oxygen percentage to below 19.5%, which creates a risk of oxygen deficiency which may cause unconsciousness or even death if the oxygen level drops below that needed to support life. As an example 5 gallons of liquid nitrogen if spilled would generate 3,475 gallons of nitrogen gas. Due to the potential to create an oxygen deficient environment an oxygen monitor may be required if calculations indicate the volume of liquid nitrogen stored in a room would create an oxygen deficient environment if released.
Below is a table outlining the effects of an oxygen deficient environment:
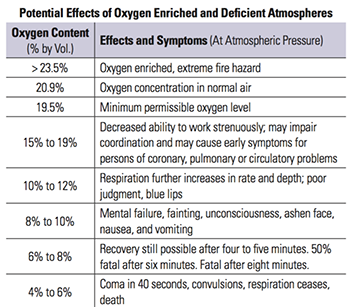
Oxygen Enrichment
Many are aware that liquid nitrogen can produce an oxygen deficient atmosphere however they are not aware that exposure of air to liquid nitrogen can also produce an oxygen enriched environment near the LN2 source. This phenomenon occurs because the boiling point of nitrogen is lower than oxygen’s, therefore there is the potential for oxygen near LN2 to also become liquefied and condense. Because liquid oxygen evaporates slower than nitrogen liquid oxygen may build up to levels which can increase the flammability of materials such as clothing near the system. Equipment containing cryogenic fluids must be kept clear of combustible materials in order to minimize the fire hazard potential. Also note that condensed oxygen in a cold trap may combine with organic material in the trap to create an explosive mixture.
Pressure Buildup and Explosions
Because of the large expansion rate of LN2, enormous pressures can build upon evaporation. Users must make sure that cryogenic liquids are never contained in a closed system. Use a pressure relief vessel or a venting lid to protect against pressure build-up.
Given the hazards of LN2 that we have just discussed, here are some recommended practices for the safe handling of LN2.
Transportation /Handling
-
Liquid nitrogen should be handled in well-ventilated areas.
-
Handle the liquid slowly to minimize boiling and splashing.
-
Use tongs to withdraw objects immersed in a cryogenic liquid - Boiling and splashing always occur when charging or filling a warm container with cryogenic liquid or when inserting objects into these liquids.
-
Do not transport liquid nitrogen in wide-mouthed glass Dewars or Dewars not protected with safety tape.
LN2 Containers
-
Use only approved containers. Impact resistant containers that can withstand the extremely low temperatures should be used. Materials such as carbon steel, plastic and rubber become brittle at these temperatures.
-
Only store liquid nitrogen in containers with loose fitting lids (Never seal liquid nitrogen in a container). A tightly sealed container will build up pressure as the liquid boils and may explode after a short time.
-
Never touch non-insulated vessels containing cryogenic liquids. Flesh will stick to extremely cold materials. Even nonmetallic materials are dangerous to touch at low temperatures.
-
Never tamper or modify safety devices such as the cylinder valve or regulator of the tank .
Storage
-
Liquid nitrogen should only be stored in well-ventilated areas (do not store in a confined space such as a cold room that is without a fresh air supply). Note: storage of liquid nitrogen at refrigerated temperatures does not slow down the evaporation process.
-
Do not store liquid nitrogen for long periods in an uncovered container.
-
Cylinders and Dewars should not be filled to more than 80% of capacity, since expansion of gases during warming may cause excessive pressure buildup.
Personal Protective Equipment
-
Eye/face protection: A full face shield over safety glasses or chemical splash goggles are recommended during transfer and handling of cryogenic liquids to minimize injuries associated with splash or explosion.
-
Skin protection: Loose-fitting thermal insulated or leather gloves, long sleeve shirts, and trousers without cuffs should be worn while handling liquid nitrogen. Safety shoes are also recommended while handling containers. Note: Gloves should be loose-fitting so they are able to be quickly removed if cryogenic liquid is spilled on them and the added space provides an insulating barrier of air between your hand and the gloves. Insulated gloves are not designed to permit the hands to be submersed in cryogenic liquid. They will only provide short-term protection from accidental contact with the liquid.
Please follow the above guidelines when using LN2 and contact our office at 274-2005 if you have any questions. |